Scale-up Challenge
A European Consortium composed of industrial and academic partners aims to optimize and further develop a novel formulation containing an enzyme used for the therapy of Fabry Disease, and establish its production at industrial scale. Fabry disease is caused by a deficiency in enzyme α-galactosidase A (GLA), which provokes the accumulation of glycosphingolipids leading to multiple organ pathology. This new formulation, obtained with the DELOS platform, is based on enzyme-loaded nanoliposomes that enhance the stability and efficacy of the enzyme. This further development requires scaling-up the manufacturing process and implement it for regulatory pre-clinical testing in order to consolidate the preclinical package.
Our Proposal
Our scientists and engineers proposed to apply a Quality by Design (QbD) approach to optimize the nanoformulation and to develop a robust process for the preparation of GLA-loaded nanoliposomes with DELOS platform, adequate for regulatory preclinical testing.
our developed
solution
Under the leadership of Nanomol Technologies, a scale-up work-package has been implemented and the following goals have been achieved:

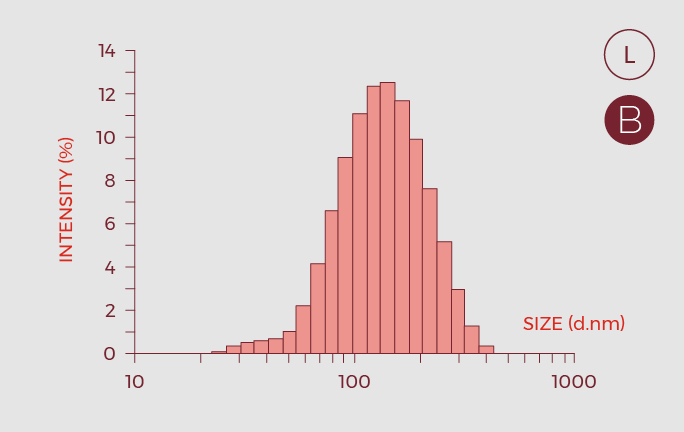
- Critical Quality Attributes (CQAs) of the nano-GLA formulation, were defined and oriented to a quality control strategy.
- Risk Analysis and Design of Experiments were performed in order to evaluate the impact of Critical Process Parameters (CPPs) and Critical Materials Atributes (CMAs) on each CQA.
- The formulation was optimized, achieving a 10-fold increase in protein concentration.
- A design space was stablished in which the product obtained by DELOS fulfils all required quality specifications
Benefits obtained
1
A nanomedicine prototype suitable for regulatory in vivo testing has been developed and demonstrated following the requirements of the pharmaceutical industry and the european regulatory authorities.
2
The formulation was successfully scaled up from the lab scale until pilot plant (liter scale).
3
High degree of process control and understanding were achieved by implementing QbD methodology.
4